|
Examples of important findings by our research on this topic
| |
 |
|
 |
|
 |
| | Spiral Growth | | | |
2D Surface Nucleation |
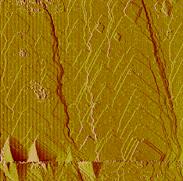
In situ observation by atomic force microscopy reveal the
mechanisms and mechanistic changes of calcite growth across a range of
supersaturation. At lower saturations,
growth is initiated solely by surface imperfections including screw
dislocations. Here, crystal
defect-originated growth, spiral growth in particular, is the only manifested
mechanism. When saturation rises
above a critical value, two-dimensional surface nucleation becomes
increasingly important.
The complexity of surface processes during spiral growth indicates that the overall growth kinetics is controlled by saturation as well as the structure of dislocation sources. This observation suggests that chemical affinity-based rate laws cannot accurately describe growth rate in these cases, and further demonstrates the need to apply caution when deducing growth mechanisms and rate laws from temporal changes in bulk solution chemistry.
Common dissolution mode, retreat of existing steps (A) and nucleation/growth of etch pits at dislocations (B) on crystal surfaces. However, etch pit formation do not take place until saturation falls below a critical value, implying that surface etching cannot be used to explain all dissolution processes.
In situ real time observations show that while the number of pit formed on crystal surfaces during dissolution increases gradually with decreasing saturation state, a leap in pit density occurs at a certain undersaturation (top), presumably indicating the transition of defect assisted pit formation (bottom left) to spontaneous pit nucleation (bottom right).
Experimental monitoring of crystal surface behavior at very near equilibrium shows simultaneous retreat (+ direction, red) and advance (- direction, blue) of steps in different crystallographic directions. This leads to the conclusion that the multiple solubilities may exist for anisotropic materials.
|
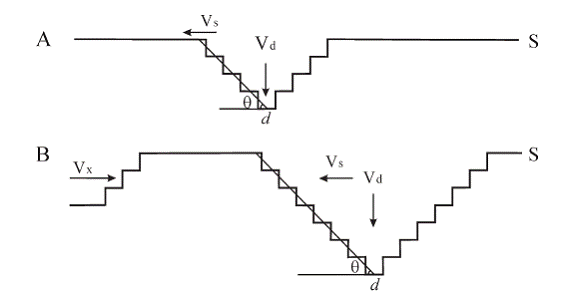
| A: D = (vd - vstanθ)t |
| B: D = (D¡¯ + (vd - vstanθ)
(l/ vx + (n-1)Dt) ¨C nh
|
Analysis of the relationship between various components of
velocity and etch pit depth. Deep pits cannot form unless the condition (vd - vstanθ)(l/ vx + (n-1)Dt) > nh is satisfied.
| 


