|
Background
Organic compounds are ubiquitously present in the atmosphere and derive from both anthropogenic and natural sources1. Out of countless numbers of organic pollutants released into the atmosphere, a number of species known as persistent, bioaccumulative, and toxic substances, (PBTs) are of a great concern1. Examples of such compounds in this category include: polyaromatic hydrocarbons, polychlorinated biphenyls, dioxins, and furans.
According to the field of studies, PBTs are present in sufficiently high levels in snow and ice in Earth’s polar regions and in high altitude localities near major urban centers1. Due to the relative transparency of ice in the UV –VIS wavelength spectral region, condensed phase photochemical reactions of PBTs may play an important role in their environmental degradation. This process, however, may not be immediately beneficial to the environment because photochemical reactions of PBTs may, in fact, produce species more toxic than their precursors1.
Potential adverse impacts of PBTs on the environment predetermined a great interest in studies of their photoreactivity. Although these studies began only recently, the results are intriguing and indicate that significant differences may exist between photochemical reaction pathways in ice and water for at least some PBT’s. For instance, photolysis of hallobenzene in ice results in halogenetated polyaromatics, while in water this photochemically induced reactions lead to the formation of phenols1.
The origins of the observed differences in outcomes of photoreactions in ice and bulk water are not completely understood1; however, the interactions with the solvent cage are likely to play a significant role. These may include limitations on the diffusion of reaction intermediates by the surrounding water matrix and reactions of the surrounding water molecules with the intermediates of such photoreactions. Thus, understanding the photochemistry of organic species in ice at temperatures near its melting point may be impossible without simultaneously taking into account variations in the physical properties of ice localized in the vicinity of an impurity. Such variations may be significant at temperatures only several degrees below the ice melting point and may even be described as molecular-scale premelting.
Although in the case of photochemical reactions in water at cryogenic temperatures, the molecular structure and properties of the H2O cage surrounding an impurity may be inferred to some degree from past experimental and theoretical studies, such information is not currently available in the case of ice undergoing premelting at temperatures near 0 °C. To our knowledge, there have been no systematic studies of structure, molecular transport properties, morphology of “quasi-liquid” in the vicinity of organic species in ice at various temperatures. Thus, despite recognition of the role of “quasi-liquid layers” in the photochemistry of ice1, current studies of photochemical reactions in this confined aqueous phase are hindered by a lack of understanding in their fundamental nature and properties.
Current interest in ice photochemistry1 makes this important research field an excellent target for FTDS exploration. While bulk studies of photochemistry in artificial snow and frozen aqueous solutions are well suited for prompt measurements of quantum yields and identifying final products of photochemical reactions, the FTDS technique will make it possible to gain molecular-level insights into the details of complex interplay between reactions and phase transitions in polycrystalline ice.
Experiments
Before outlining the particulars of planned photochemical experiments, we would like to emphasize the unique capabilities offered by FTDS in studies of ice photochemistry. First, in FTDS experiments the microstructure of aqueous films can easily be varied and controlled during deposition at cryogenic temperatures. Second, due to near collision-free desorption at higher temperatures, chemical species (reaction products and intermediates) can be directly analyzed by mass spectrometry without taking into account gas phase processes. Third, because in a rapidly evaporating aqueous film each elementary reaction step proceeds in competition with desorption, it is potentially feasible to “preserve” many of the short-lived intermediates for direct detection with a mass spectrometer. Fourth, in cases where a reaction results in hyperthermal products, it may be possible to distinguish between products of surface and bulk reactions by using FTDS in combination with TOF measurements.
In addition to the general strengths of the FTDS technique, we must also emphasize the small size of the crystallites in our thin ice film as a particular advantage of this approach, which has direct relevance to current studies of environmentally relevant photochemical reactions in polycrystalline ice. Due to the sensitivity limits of contemporary analytical techniques, studies of photochemistry in bulk ice are conducted under conditions where concentrations of dopant species at grain boundaries may be extremely high. This is a direct consequence of a relatively large average size of ice crystallites (fraction of a millimeter) in frozen of aqueous solutions, which leads to segregation-driven enhancement of grain boundary concentrations of organic species by many orders of magnitude1.
Because our polycrystalline ice films are prepared by homogeneous crystallization of amorphous solid water during fast heating and because the typical timescale of our FTDS experiments is in the millisecond range, the average size of the crystallite is extremely small2,3. Indeed, calculations based on known kinetics of grain growth results in a value of less than 30 nanometers for an average diameter of the crystallites in the polycrystalline ice films in FTDS studies2,3. This estimate is corroborated by our studies of morphological dynamics of rapidly vaporizing ice films4, which shows that perhaps as much as 10% of water molecules in our polycrystalline ice samples may reside at the grain boundaries. In other words, due to a large volume fraction occupied by grain boundaries in our polycrystalline ice samples, the dopant concentration enhancement effect in FTDS studies will be minimal, thus, making it possible to conduct photochemical experiments under environmentally relevant conditions despite a high overall concentration of organic species.
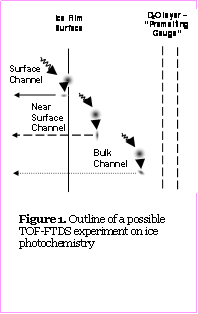
An example of an experimental arrangement for a photolysis experiment is shown in Figure 1. A 50-100 nm region of the ice film near its surface will be doped with an organic species of interest, such as chlorobenzene. A 100 nm thick D2O/C6H5Cl layer will positioned in the films bulk of H2O ice and will serve as a “premelting gauge”, i.e., will be used to determine the extent of isotopic exchange at a particular temperature and, thus, to estimate the extent of premelting during the FTDS experiment. The mole fraction of C6H5Cl in doped ice will be less that 0.01, which, according to our estimates of grain boundary densities, will result in average separation between chlorobenzene molecules of more than several nanometers. The temperature of the film will be brought to a value near 0 °C.
After the onset of isothermal vaporization, the complex structured film shown in Fig. 11 will be subjected to a UV pulse from an excimer laser. The laser pulse will also initiate a data acquisition sequence in which signals from QMS I and QMS II will be recorded as function of time. Thus, the resulting fast thermal desorption spectra and TOF distributions of products and intermediates of the reactions will be measured. In addition to simple identification of the photochemical products, the diverse data obtained in FTDS-TOF experiment will make it possible to distinguish between surface, near surface, and bulk photolysis channels (if they are different). Finally, the exchange in the D2O layer will provide information on the extent of possible premelting in the film.
References:
1. A. M. Grannas et al. “An overview of snow photochemistry: evidence, mechanisms and impacts”, Atmospheric Chemistry and Physics, 7, 4329, (2007)
2. Haiping Lu Stephanie A. McCartney, and Vlad Sadtchenko, J. Chem. Phys, submitted (2007) PDF 3. Haiping Lu, Stephanie A. McCartney, and Vlad Sadtchenko, J. Chem. Phys. 127, 184701 (2007)
4. H. P. Lu, S. A. McCartney, M. Chonde, D. Smyla, and V. Sadtchenko, “Fast Thermal Desorption Spectroscopy Study of Morphology and Vaporization Kinetics of Polycrystalline Ice Films”, J. Chem. Phys., 125, 044709, (2006).
|
|
TOF-FTDS studies of Ice photochemistry |
|
Vlad Sadtchenko, GW Chemistry |