|
FTDS studies of H/D exchange reactions in polycrystalline ice near its melting point - a sensitive probe of grain boundary premelting
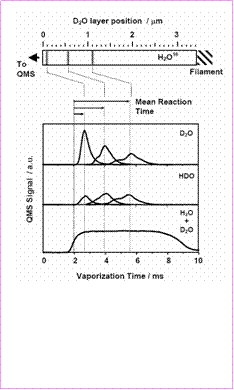
In order to demonstrate that FTDS can be used successfully to study reactions in volatile polycrystalline materials and to gain insights into nanoscale molecular transport, and phase transitions in polycrystalline ice we have conducted extensive studies of H/D exchange kinetics in layered H2O/D2O/H2O films at temperatures between -20 and -1 °C1,2. Figure 1 illustrates our approach.
As shown in the Figure 1, a thin layer of D2O, initially positioned at a distance away from the film surface, evolves from the ice film only after vaporization of the overlaying H2O16. The time interval from the onset of H2O vaporization to the appearance of the D2O vaporization peak, i.e., the mean residence time of D2O in the bulk of the H2O film, gives the mean reaction time for H2O/D2O isotopic exchange. Positioning the D2O layer further away from the surface, i.e. increasing the mean reaction time, results in a gradual decrease of the magnitude of the D2O peak. The decrease in the D2O peak is accompanied by a gradual increase in the HDO yield signifying rapid conversion of D2O to HDO.
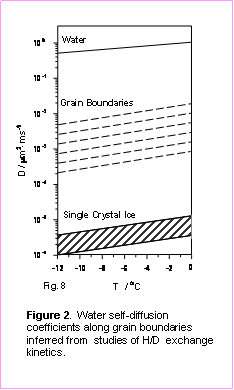
Integrating the HDO and D2O spectra in Fig. 1 over vaporization time makes it possible to infer kinetics of the isotope exchange reaction in polycrystalline ice. As we show in our recent publications1,2, the H/D exchange kinetics measured in FTDS experiments are defined by the rate of molecular interdiffusion between polycrystalline H2O and D2O layers. Thus, using the isotopic exchange reaction as a probe of molecular transport, we were able to determine the effective water-self diffusion coefficients in pure polycrystalline ice in a temperature range from -12 to -1 °C. Combining H/D exchange data with previously developed theoretical models of diffusive transport in polycrystalline materials, we were able to infer the water self-diffusion coefficients at the grain boundaries at various temperatures. The results of these studies are summarized in Figure 2.
According to our analysis, the effective activation energy of water self-diffusion along the grain boundaries is 69 ± 5 kJ·mol-1, i.e., near the greatest measured value for water self-diffusion in single crystal ice (70 kJ·mol-1), and much higher that the Ea in bulk water or in the surface “quasi-liquid layer” (25-30 kJ·mol-1). In other words, the inferred value of the effective activation energy for water self-diffusion along the grain boundaries in a fine polycrystalline ice excludes the possibility of the formation of liquid-like inclusions in the bulk of pure polycrystalline ice at temperatures up to -2 °C.
The diffusivity data in Fig. 2 makes it possible to gain insights into the nature of condensed aqueous phase at grain boundaries in polycrystalline ice at various temperatures. For example, the high value of the activation energy for water self-diffusion at the grain boundaries in pure ice excludes the possibility of the formation of a “quasi-liquid” anywhere in the ice sample even at temperatures as high as one degree below ice melting point. Consequently, the grain boundaries in pure ice should not be considered as either liquid channels or as fissures in polycrystalline sample bulk.
Studies of molecular transport and structure of pure polycrystalline ice are important for gaining a fundamental understanding of the general physical and chemical properties of water and other hydrogen bonded solids and liquids. Nevertheless, in the environment, condensed aqueous phases are rarely free of impurities. In order to improve understanding of environmentally relevant phenomena in ice, we have conducted FTDS investigations of molecular transport in polycrystalline ice doped with HCl. The essential findings of this study are described immediately below.
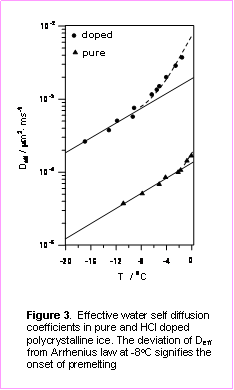
Figure 3 compared the effective water self-diffusion coefficients, Deff, in pure films and those doped with HCl, which we inferred from FTDS measurements of H/D exchange kinetics. As shown in the figure, in the case of pure polycrystalline ice, the Deff dependence on temperature is consistent with the Arrhenius law up to -2 °C. However, addition of 0.04 % (by mass) of HCl to the polycrystalline ice sample not only increases the apparent water self-diffusion coefficient, but also results in a pronounced deviation of the Deff from Arrhenius law at temperatures near -8 °C. According, to our analysis2, the change in kinetics of molecular transport occurs due to formation of liquid-like inclusion in the bulk of doped polycrystalline ice in the presence of HCl.
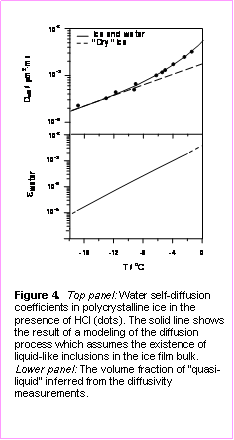
Combining the diffusivity data obtained in our FTDS experiments with a simple phenomenological model of diffusion in non-homogeneous media, we were able to determine the volume fraction occupied by “quasi-liquid” in the bulk of doped polycrystalline ice sample at various temperatures. The unexpected results of this analysis are illustrated in Figure 6, which shows that the volume fraction of liquid in HCl doped ice is relatively small even at temperature as high as -2 °C. Pending validation by future experiments, we argue that our estimates of the extent of the premelting exclude a possibility of the formation of a “quasi-liquid” layer at the grain boundaries and that the formation of liquid veins at the grain boundary intersections is the most likely explanation for the observed temperature dependence of water self-diffusivity in doped polycrystalline ice. We emphasize that these important case studies represent just the first steps in a program of extensive research into aqueous phase transition phenomena. In the future, we will continue our studies of water self-diffusion in doped polycrystalline ice, which will include investigations of premelting as a function of impurity concentrations. In addition to HCl, we will study ice premelting in the presence of HI, HBr, HNO3, SO2, NH4 and other inorganic species.
References:
1. Haiping Lu Stephanie A. McCartney, and Vlad Sadtchenko, J. Chem. Phys, submitted (2007), and references therein PDF
2. “Haiping Lu, Stephanie A. McCartney, and Vlad Sadtchenko, J. Chem. Phys. 127, 184701 (2007) |
|
Figure 1. Isothermal vaporization spectra of HDO and D2O from a sandwich -like H2O/D2O/H2O film at -5 oC. The diagram on top of the figure shows initial position of D2O layer.
|
|
Vlad Sadtchenko, GW Chemistry |